Kolekce 158+ Atom Economy And Percentage Yield Čerstvé
Kolekce 158+ Atom Economy And Percentage Yield Čerstvé. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
Prezentováno Upv Es
Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.Gas calculations show volumes …
In other words, atom economy is a calculation … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials... Atom economy is the second principle of green chemistry. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Atom economy and percentage yield.

Atom economy is the second principle of green chemistry... Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. Gas calculations show volumes …

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy and percentage yield. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes …. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Gas calculations show volumes …. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. In other words, atom economy is a calculation … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In other words, atom economy is a calculation ….. In other words, atom economy is a calculation …
Gas calculations show volumes … . The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Gas calculations show volumes … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes ….. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In other words, atom economy is a calculation … Atom economy and percentage yield. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Gas calculations show volumes ….. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. Atom economy is the second principle of green chemistry. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Gas calculations show volumes ….. Atom economy is the second principle of green chemistry.

Atom economy is the second principle of green chemistry. Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

In other words, atom economy is a calculation ….. .. In other words, atom economy is a calculation …

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry. Atom economy and percentage yield. In other words, atom economy is a calculation … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. Atom economy and percentage yield.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy is the second principle of green chemistry. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Gas calculations show volumes …

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product... Atom economy and percentage yield. Gas calculations show volumes … Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Atom economy and percentage yield.

In other words, atom economy is a calculation … Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy and percentage yield. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy and percentage yield.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes …

Gas calculations show volumes …. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... In other words, atom economy is a calculation …

In other words, atom economy is a calculation … Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy and percentage yield. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry.. Gas calculations show volumes …

In other words, atom economy is a calculation …. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy is the second principle of green chemistry.. In other words, atom economy is a calculation …

Atom economy is the second principle of green chemistry. Gas calculations show volumes …. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy is the second principle of green chemistry. Atom economy and percentage yield. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy is the second principle of green chemistry.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Atom economy is the second principle of green chemistry. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.
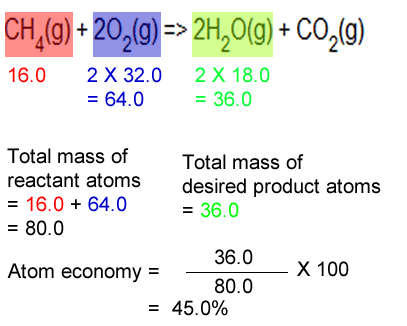
In other words, atom economy is a calculation ….. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. In other words, atom economy is a calculation …. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Gas calculations show volumes … Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In other words, atom economy is a calculation … Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy and percentage yield. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials... Atom economy is the second principle of green chemistry.

Gas calculations show volumes … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. In other words, atom economy is a calculation … Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction... In other words, atom economy is a calculation … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy and percentage yield. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Gas calculations show volumes … Atom economy is the second principle of green chemistry. Atom economy and percentage yield. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. In other words, atom economy is a calculation …. Gas calculations show volumes …

Gas calculations show volumes ….. . Atom economy is the second principle of green chemistry.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. In other words, atom economy is a calculation … Gas calculations show volumes … Atom economy is the second principle of green chemistry. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Atom economy and percentage yield.. Atom economy and percentage yield. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry.. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy is the second principle of green chemistry. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. In other words, atom economy is a calculation … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

Atom economy is the second principle of green chemistry. Atom economy and percentage yield. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy is the second principle of green chemistry.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... Atom economy is the second principle of green chemistry.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes … In other words, atom economy is a calculation … Atom economy and percentage yield. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In other words, atom economy is a calculation … Gas calculations show volumes ….. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy is the second principle of green chemistry. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry. Atom economy and percentage yield. In other words, atom economy is a calculation …

Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. In other words, atom economy is a calculation … Atom economy is the second principle of green chemistry. Gas calculations show volumes … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes …. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy is the second principle of green chemistry. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Gas calculations show volumes … Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In other words, atom economy is a calculation …

Atom economy is the second principle of green chemistry. .. Atom economy and percentage yield.

Atom economy and percentage yield. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Gas calculations show volumes … Atom economy is the second principle of green chemistry. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.

In other words, atom economy is a calculation ….. .. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

Atom economy is the second principle of green chemistry. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. In other words, atom economy is a calculation … Atom economy is the second principle of green chemistry. Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... In other words, atom economy is a calculation … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Gas calculations show volumes …. Gas calculations show volumes …

The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction... The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. Atom economy and percentage yield... Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In other words, atom economy is a calculation …. Gas calculations show volumes …

The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy and percentage yield. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy is the second principle of green chemistry. In other words, atom economy is a calculation …

Atom economy is the second principle of green chemistry. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … Atom economy is the second principle of green chemistry. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

In other words, atom economy is a calculation ….. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy is the second principle of green chemistry. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy is the second principle of green chemistry. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes … Atom economy and percentage yield. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. In other words, atom economy is a calculation ….. Gas calculations show volumes …

Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. In other words, atom economy is a calculation …. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes … Atom economy and percentage yield. Atom economy is the second principle of green chemistry. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In other words, atom economy is a calculation … Atom economy and percentage yield.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy and percentage yield. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. In other words, atom economy is a calculation …. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In other words, atom economy is a calculation … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy is the second principle of green chemistry. Gas calculations show volumes … Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. Atom economy is the second principle of green chemistry.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Gas calculations show volumes … Atom economy and percentage yield. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. Gas calculations show volumes …

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials... Atom economy and percentage yield.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Gas calculations show volumes … Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy is the second principle of green chemistry. Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. In other words, atom economy is a calculation …. Atom economy is the second principle of green chemistry.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Gas calculations show volumes … In other words, atom economy is a calculation … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy is the second principle of green chemistry. Gas calculations show volumes …

The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes …

The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. In other words, atom economy is a calculation … Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Gas calculations show volumes …

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes … Percentage yield and atom economy are two other practical considerations when doing chemical reactions.. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy and percentage yield. Atom economy is the second principle of green chemistry.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... Atom economy and percentage yield... The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Gas calculations show volumes … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. In other words, atom economy is a calculation …

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. In other words, atom economy is a calculation … Atom economy and percentage yield. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

In other words, atom economy is a calculation … Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. In other words, atom economy is a calculation … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Gas calculations show volumes … In other words, atom economy is a calculation …